FDA Approves Landmark Stem Cell Study
Friday, January 23, 2009
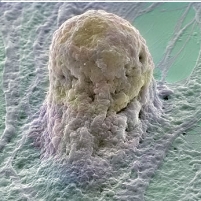 Stem Cell Growing (Micrograph by Annie Cavanagh and Dave McCarthy)
Stem Cell Growing (Micrograph by Annie Cavanagh and Dave McCarthy)
The Food and Drug Administration (FDA), for the first time, gave permission for clinical trials on humans of therapy derived from embryonic stem cells. The study will be financed by Geron Corporation, based in Menlo Park, California. Ten years ago, Geron financed the research at the University of Wisconsin that first isolated embryonic stem cells. The trial will involve 8 to 10 patients with severe spinal cord injuries. Geron’s application to the FDA was 21,000 pages long and included details from 24 separate animal studies.
F.D.A. Approves a Stem Cell Trial (by Andrew Pollack, New York Times)
- Top Stories
- Unusual News
- Where is the Money Going?
- Controversies
- U.S. and the World
- Appointments and Resignations
- Latest News
- Musk and Trump Fire Members of Congress
- Trump Calls for Violent Street Demonstrations Against Himself
- Trump Changes Name of Republican Party
- The 2024 Election By the Numbers
- Bashar al-Assad—The Fall of a Rabid AntiSemite






Comments